investigations
background
Boiling Fluids On the Seafloor: Development of Black Smoker Chimneys
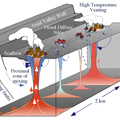
The spectacular development of vigorously venting, 350°– 407°C, black smokers on the ocean floor is fueled by circulation and heating of seawater within the oceanic crust (Figure 1). In areas such as the Endeavour, convection of heated seawater, or hydrothermal fluids, is driven by heat from magma chambers at depths of ~2 km beneath the seafloor (Figure 2).
As cool, dense seawater migrates deep within the oceanic crust along networks of cracks and fractures, important chemical and physical changes take place. As the fluids are heated to temperatures of 350°C to 450°C, mineral-fluid reactions result in quantitative loss of magnesium from solution and coupled release of protons. These reactions cause the resulting hydrothermal fluids to become acidic (pH 2-5). The combination of heat and increased acid leaches elements such as copper, zinc, iron, sulfur, and silica from the basaltic host rocks.
Gases such as carbon dioxide (CO2) and helium (He) are added to the hydrothermal fluid by direct release from magma chambers (magmatic gases) or by leaching from the enclosing host rock. Hydrogen (H2) is formed during cracking of the rocks at high temperature through mineral-fluid reactions. CO2, H2, H2S, and CH4 are important in geobiological processes because they form critical nutrients and energy sources for microbiological development at shallow crustal levels. As the downwelling fluids reach temperatures of 400°C or more, they become extremely buoyant, their viscosity and density decrease significantly, and their ability to carry heat increases. Similar to the way water changes into steam when it is heated in a pan on the stove, the now buoyant, metal-rich fluids rise up through fractures in the seafloor, drawing in cooler fluids in their wake, and a circulation system develops (Figure 3).
As the acidic, metal- and volatile-rich, 350°C–400°C fluids are buoyantly ejected through cracks in the seafloor into the overlying water column, they mix turbulently with oxygen-rich, cold (2°C) seawater. This drastic temperature change causes very fine-grained iron-, copper-, and zinc-sulfides, such as pyrite, chalcopyrite, and sphalerite, and other sulfide-bearing minerals to rapidly crystallize from the hydrothermal fluids. Much of the fine-grained sulfide particles are carried upward by jet-like plumes at velocities of a meter/second or more. Sulfide and sulfate minerals not carried away in the hydrothermal plumes are deposited around the opening of the vent, causing the vent to grow upward and outward over time.