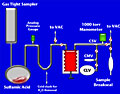
investigations
background
Chemistry
The Chemistry of Hydrothermal Vent Fluids
Hot Rocks and Seawater
Vent fluids form when seawater flows down into the Earth and reaches magma chambers, very hot areas in the rock far below the ocean bottom. The seawater is heated and reacts chemically when it comes into contact with the hot rock.
This chemically altered fluid is less dense than the original seawater that made its way to the magma chamber. Flowing upward, finding channels, the new fluid emerges at the seafloor as underwater hot springs, forming the hydrothermal vent fields such as Endeavour. There, on the seafloor, the hot hydrothermal vent fluid mixes with the cold seawater and minerals precipitate to form sulfide chimneys.
Constantly Changing Chemistry
According to the magazine Oceanus the chemistry of hydrothermal fluids starts changing when the seawater percolates into the upper layer of the crust. This is referred to as the recharge zone. The seawater reacts with the basaltic rocks and causes a partial oxidation, which strips seawater of its oxygen content. Iron in the rocks is converted to iron oxides and hydroxides. As seawater breaks down the original rock minerals, potassium and other alkali elements are transferred from seawater to the rocks. As this water flows deeper in the crust, the temperature rises and hydroxyl ions are removed from the fluid. The resultant fluid is now acidic (the fluid has a lower pH), which allows alkali and alkaline earth metals to be redirected into the fluid.
The reaction zone in which the fluids will penetrate deeper involves high temperature water-rock reactions. These reactions vary based on how deep the heat source is. A variety of minerals are produced at this depth, but the main reaction that occurs here is the absorption of metals and sulfur, which accounts for the large sulfide deposits that precipitate at vent sites on the seafloor.
The upflow zone is where the heated water rises quickly towards the seafloor, picking up more metals and sulfur and leaving behind magnesium. Hydrogen and methane are also absorbed at this time. When the hot fluid exits from the seafloor it mixes with the seawater, and forms black smokers as the metal sulfides precipitate out in the cold water. If it slowly diffuses at a lower temperature, then the metallic sulfides will stay beneath the crust, sulfates will emerge and white smokers are discharged.